Summary
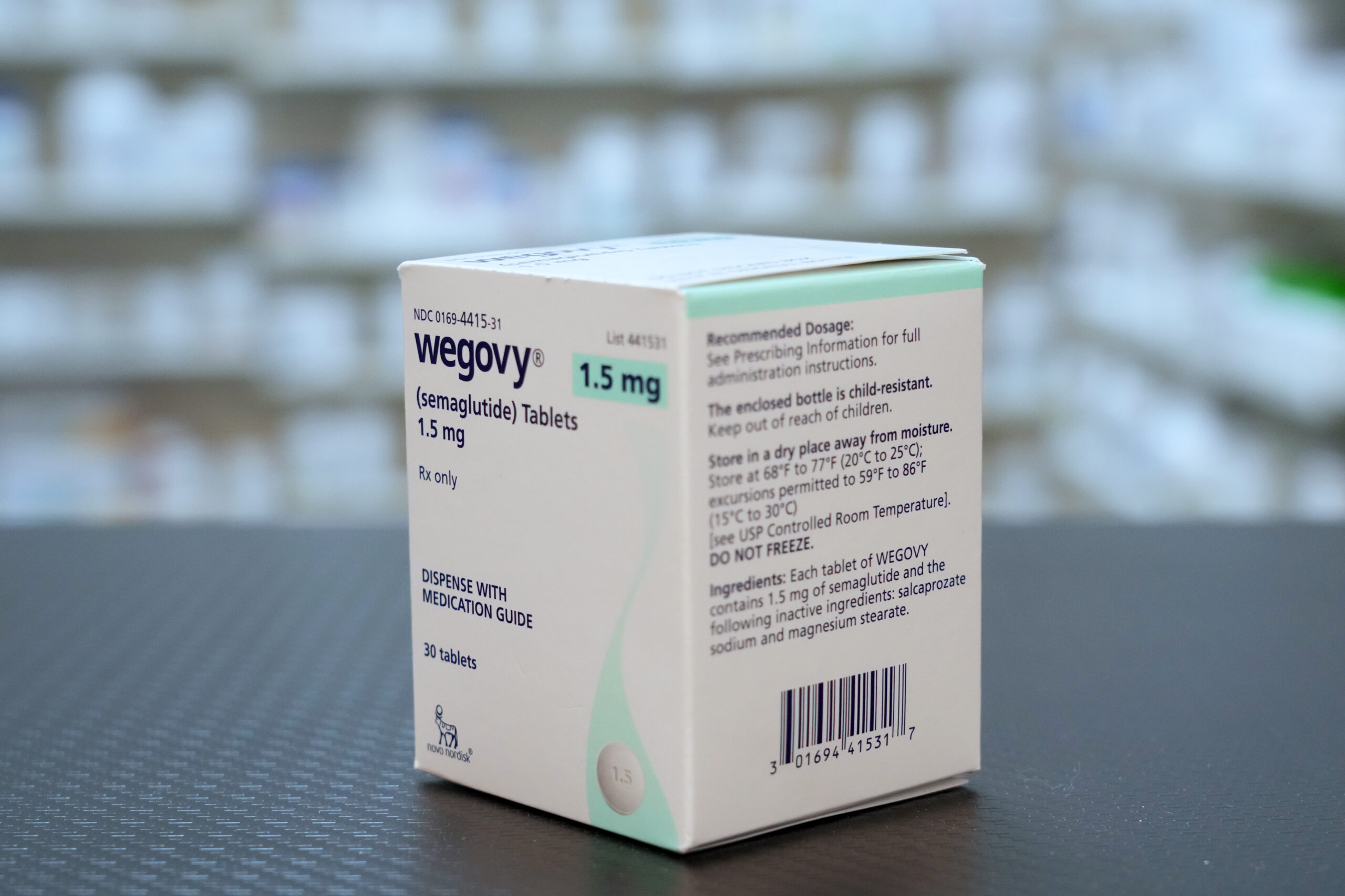
Novo Nordisk is suing Hims & Hers Health Inc. for making knock-offs of its obesity medicines, even as Hims scrapped plans to sell a copycat version of the Wegov…
Source: claimsjournal.com

AI News Q&A (Free Content)
Q1: What are the main claims of Novo Nordisk's lawsuit against Hims & Hers regarding the obesity drug Wegovy?
A1: Novo Nordisk is suing Hims & Hers for allegedly selling unauthorized versions of its obesity drug, Wegovy. The lawsuit claims that Hims & Hers marketed these cheaper, compounded versions without undergoing the FDA's rigorous approval process, potentially endangering patient safety. Novo Nordisk seeks a permanent ban on these sales and is pursuing damages for patent infringement.
Q2: How has the recent legal action impacted the stock prices of Novo Nordisk and Hims & Hers?
A2: Following the announcement of the lawsuit, Novo Nordisk's shares rose by over 5%, reflecting investor confidence in the company's legal position. In contrast, Hims & Hers experienced a significant drop in its stock value by 21%, indicating market concerns over the potential financial and reputational impact of the lawsuit.
Q3: What is semaglutide, and how is it used in treating obesity and diabetes?
A3: Semaglutide is the active ingredient in Novo Nordisk's Wegovy, approved for weight loss, and Ozempic, used for managing Type 2 diabetes. Semaglutide works by mimicking a hormone that targets areas of the brain involved in regulating appetite and food intake, thereby assisting in weight reduction and glycemic control.
Q4: What are the economic trends and market projections for anti-obesity medications like Wegovy?
A4: The global anti-obesity medication market, valued at USD 6.62 billion in 2023, is expected to grow to USD 77.24 billion by 2030, with a CAGR of 31.66%. This growth is driven by increasing obesity rates and demand for effective treatments. North America leads the market due to high obesity prevalence and supportive policies.
Q5: How does the use of semaglutide impact patients with heart failure and obesity according to recent studies?
A5: A recent study demonstrated that semaglutide significantly improved symptoms and physical limitations in patients with heart failure with preserved ejection fraction and obesity. Patients experienced a notable reduction in body weight and improved physical capacity, highlighting semaglutide's potential beyond weight loss.
Q6: What role do regulatory bodies like the FDA play in the approval and monitoring of medications similar to Wegovy?
A6: The FDA ensures that drugs like Wegovy meet stringent safety, efficacy, and quality standards before approval. This involves rigorous clinical trials to evaluate the drug's effects and safety profile. The FDA also monitors post-market data to ensure ongoing compliance with safety standards, protecting public health.
Q7: What are the challenges and considerations in the economic feasibility of obesity treatment drugs from a healthcare perspective?
A7: The economic feasibility of obesity treatments involves balancing drug efficacy, patient accessibility, and healthcare costs. While effective drugs like Wegovy are in high demand, their costs pose challenges for healthcare systems and patients. Policymakers must consider strategies to make these treatments accessible while managing financial burdens.
References:
- Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity