Summary
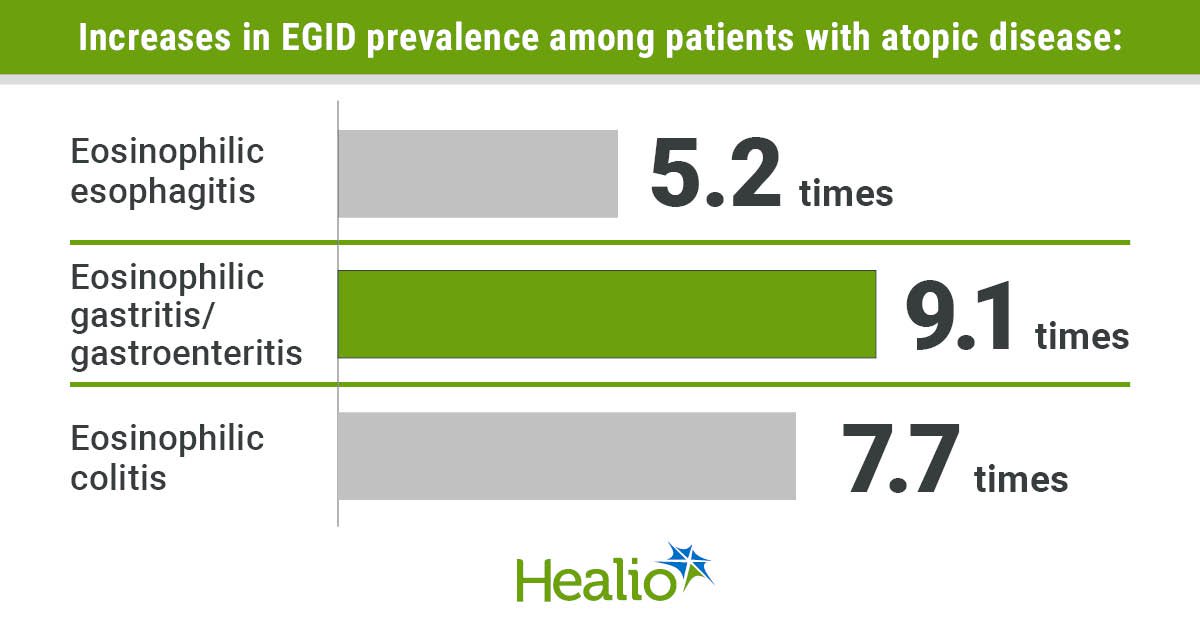
Eosinophilic gastrointestinal diseases are exponentially more prevalent among patients with atopic diseases compared with the general population, according to a study published in Annals of Allergy, Asthma & Immunology.
Source: Healio

AI News Q&A (Free Content)
Q1: What are eosinophilic gastrointestinal diseases and how do they relate to atopic disorders?
A1: Eosinophilic gastrointestinal diseases (EGIDs) are characterized by an excessive number of eosinophils, a type of white blood cell, in the gastrointestinal tract. These conditions are often associated with atopic disorders such as asthma, eczema, and allergic rhinitis. Studies have shown that patients with atopic disorders are more likely to develop EGIDs due to shared inflammatory pathways and genetic predispositions.
Q2: How prevalent are eosinophilic gastrointestinal diseases among patients with atopic conditions compared to the general population?
A2: The prevalence of eosinophilic gastrointestinal diseases is significantly higher among patients with atopic conditions. For instance, eosinophilic esophagitis is more common among individuals with two or more atopic diseases, with the prevalence increasing from 1.6% with two atopic diseases to 4.2% with four or more. This suggests a strong link between atopic diseases and the development of EGIDs.
Q3: What are the potential implications of eosinophilic gastrointestinal diseases on diagnosis and treatment?
A3: The close association between atopic diseases and eosinophilic gastrointestinal diseases implies that early assessments for EGIDs in patients with atopic conditions may reduce delays in diagnosis. This could lead to more timely treatment and management strategies, potentially improving patient outcomes by addressing both eosinophilic and atopic conditions simultaneously.
Q4: What recent advancements have been made in the genetic understanding of eosinophilic esophagitis?
A4: Recent advancements in genetic research have identified multiple genetic susceptibilities linked to eosinophilic esophagitis (EoE). Genome-wide association studies have expanded knowledge on the polygenic risk scores, highlighting genetic markers that contribute to the condition. This research is crucial for developing targeted genetic therapies and personalized treatment strategies.
Q5: How is artificial intelligence being utilized to improve the diagnosis of eosinophilic esophagitis?
A5: Artificial intelligence platforms have been developed to enhance the diagnosis of eosinophilic esophagitis by analyzing biopsy images. These AI systems infer spatial biomarkers and assess histological severity with high accuracy, sensitivity, and specificity. The use of AI in this context streamlines diagnosis processes, potentially leading to more accurate and quicker patient assessments.
Q6: What role do heat shock proteins play in the treatment of atopic dermatitis, and how might this relate to eosinophilic conditions?
A6: Heat shock proteins, particularly from the HSP90 family, have been identified as promoters of inflammation in atopic dermatitis. Inhibitors like 17-AAG have shown promise in reducing inflammation and partially restoring microbial balance. These findings suggest that similar therapeutic approaches might be beneficial in managing eosinophilic conditions by targeting inflammatory pathways.
Q7: What challenges exist in diagnosing eosinophilic esophagitis, and how might they be addressed?
A7: Diagnosing eosinophilic esophagitis is challenging due to non-specific symptoms and histopathologic findings. The condition often requires invasive procedures like endoscopy for confirmation. However, advancements in AI and genetic research are paving the way for less invasive, more precise diagnostic tools that can help in early detection and management of the disease.
References:
- Harnessing Artificial Intelligence to Infer Novel Spatial Biomarkers for the Diagnosis of Eosinophilic Esophagitis
- Multi-trait Analysis of GWAS Expands Eosinophilic Esophagitis Genetic Susceptibility and Polygenic Risk Scores